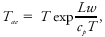Equivalent temperature: Difference between revisions
From Glossary of Meteorology
No edit summary |
m (Rewrite with Template:Term and clean up) |
||
| Line 1: | Line 1: | ||
{{Term | |||
|Display title=equivalent temperature | |||
{{ | |Definitions={{Definition | ||
{{ | |Num=1 | ||
|Meaning=#Isobaric equivalent temperature: The [[temperature]] that an [[air parcel]] would have if all [[water vapor]] were condensed at constant [[pressure]] and the [[enthalpy]] released from the [[vapor]] used to heat the air: | |||
|Explanation=<blockquote>[[File:ams2001glos-Ee58.gif|link=|center|ams2001glos-Ee58]]</blockquote> where ''T''<sub>''ie''</sub> is the isobaric equivalent temperature, ''T'' the [[temperature]], ''w'' the [[mixing ratio]], ''L'' the [[latent heat]], and ''c''<sub>''p''</sub> the [[specific heat]] of air at constant pressure. This process is physically impossible in the [[atmosphere]].<br/> | |||
#Adiabatic equivalent temperature (also known as pseudoequivalent temperature): The [[temperature]] that an [[air parcel]] would have after undergoing the following process: [[dry-adiabatic process|dry-adiabatic]] expansion until saturated; [[pseudoadiabatic expansion]] until all moisture is precipitated out; dry- adiabatic compression to the initial [[pressure]].<br/> This is the equivalent temperature as read from a thermodynamic [[chart]] and is always greater than the isobaric equivalent temperature: <blockquote>[[File:ams2001glos-Ee59.gif|link=|center|ams2001glos-Ee59]]</blockquote> where ''T''<sub>''ae''</sub> is the adiabatic equivalent temperature. | |||
}} | |||
}} | |||
# | |||
# | |||
Latest revision as of 07:16, 29 March 2024
- Isobaric equivalent temperature: The temperature that an air parcel would have if all water vapor were condensed at constant pressure and the enthalpy released from the vapor used to heat the air:
where Tie is the isobaric equivalent temperature, T the temperature, w the mixing ratio, L the latent heat, and cp the specific heat of air at constant pressure. This process is physically impossible in the atmosphere.
- Adiabatic equivalent temperature (also known as pseudoequivalent temperature): The temperature that an air parcel would have after undergoing the following process: dry-adiabatic expansion until saturated; pseudoadiabatic expansion until all moisture is precipitated out; dry- adiabatic compression to the initial pressure.
This is the equivalent temperature as read from a thermodynamic chart and is always greater than the isobaric equivalent temperature:
where Tae is the adiabatic equivalent temperature.