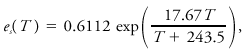Clausius-clapeyron equation: Difference between revisions
m (Rewrite with Template:Term and clean up) |
Ametsocadmin (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{DISPLAYTITLE:Clausius-Clapeyron equation}} | |||
(''Also called'' Clapeyron equation, Clapeyron–Clausius equation.) The differential equation relating [[pressure]] of a substance to [[temperature]] in a system in which two phases of the substance are in [[equilibrium]].<br/> Two general expressions are <blockquote>[[File:ams2001glos-Ce17.gif|link=|center|ams2001glos-Ce17]]</blockquote> where ''p'' is the pressure, ''T'' the temperature, δ''s'' the difference in [[specific entropy]] between the phases, δ''v'' the difference in [[specific volume]] between the two phases, and ''L'' the [[latent heat]] of the [[phase change]]. The form most familiar in meteorology, related to the phase change between [[water vapor]] and liquid water, is obtained after some approximations as <blockquote>[[File:ams2001glos-Ce18.gif|link=|center|ams2001glos-Ce18]]</blockquote> where ''e''<sub>''s''</sub> is the [[saturation vapor pressure]] of water, ''L''<sub>''v''</sub> the latent heat of [[vaporization]], and ''R''<sub>''v''</sub> the [[gas constant]] for water vapor. A similar relation for the saturation vapor pressure in contact with an [[ice]] surface is obtained by replacing the latent heat of vaporization by that of [[sublimation]]. These equations may be integrated to obtain explicit relationships between ''e''<sub>''s''</sub> and ''T'', given known values at some point. The most empirically accurate relationships differ slightly from results so obtained. An expression believed accurate to 0.3% for -35°C < ''T'' < 35°C is given by Bolton as <blockquote>[[File:ams2001glos-Ce19.gif|link=|center|ams2001glos-Ce19]]</blockquote> where ''T'' is temperature in °C and [[vapor pressure]] is in kPa.<br/> Iribarne, J. V., and W. L. Godson. 1981. ''Atmospheric Thermodynamics''. D. Reidel, p. 65. <br/> Bolton, D. 1980. The computation of equivalent potential temperature. ''Mon. Wea. Rev.'', '''108'''. 1046–1053. <br/> | (''Also called'' Clapeyron equation, Clapeyron–Clausius equation.) The differential equation relating [[pressure]] of a substance to [[temperature]] in a system in which two phases of the substance are in [[equilibrium]].<br/> Two general expressions are <blockquote>[[File:ams2001glos-Ce17.gif|link=|center|ams2001glos-Ce17]]</blockquote> where ''p'' is the pressure, ''T'' the temperature, δ''s'' the difference in [[specific entropy]] between the phases, δ''v'' the difference in [[specific volume]] between the two phases, and ''L'' the [[latent heat]] of the [[phase change]]. The form most familiar in meteorology, related to the phase change between [[water vapor]] and liquid water, is obtained after some approximations as <blockquote>[[File:ams2001glos-Ce18.gif|link=|center|ams2001glos-Ce18]]</blockquote> where ''e''<sub>''s''</sub> is the [[saturation vapor pressure]] of water, ''L''<sub>''v''</sub> the latent heat of [[vaporization]], and ''R''<sub>''v''</sub> the [[gas constant]] for water vapor. A similar relation for the saturation vapor pressure in contact with an [[ice]] surface is obtained by replacing the latent heat of vaporization by that of [[sublimation]]. These equations may be integrated to obtain explicit relationships between ''e''<sub>''s''</sub> and ''T'', given known values at some point. The most empirically accurate relationships differ slightly from results so obtained. An expression believed accurate to 0.3% for -35°C < ''T'' < 35°C is given by Bolton as <blockquote>[[File:ams2001glos-Ce19.gif|link=|center|ams2001glos-Ce19]]</blockquote> where ''T'' is temperature in °C and [[vapor pressure]] is in kPa.<br/> Iribarne, J. V., and W. L. Godson. 1981. ''Atmospheric Thermodynamics''. D. Reidel, p. 65. <br/> Bolton, D. 1980. The computation of equivalent potential temperature. ''Mon. Wea. Rev.'', '''108'''. 1046–1053. <br/> | ||
Latest revision as of 10:26, 19 April 2024
(Also called Clapeyron equation, Clapeyron–Clausius equation.) The differential equation relating pressure of a substance to temperature in a system in which two phases of the substance are in equilibrium.
Two general expressions are
where p is the pressure, T the temperature, δs the difference in specific entropy between the phases, δv the difference in specific volume between the two phases, and L the latent heat of the phase change. The form most familiar in meteorology, related to the phase change between water vapor and liquid water, is obtained after some approximations as
where es is the saturation vapor pressure of water, Lv the latent heat of vaporization, and Rv the gas constant for water vapor. A similar relation for the saturation vapor pressure in contact with an ice surface is obtained by replacing the latent heat of vaporization by that of sublimation. These equations may be integrated to obtain explicit relationships between es and T, given known values at some point. The most empirically accurate relationships differ slightly from results so obtained. An expression believed accurate to 0.3% for -35°C < T < 35°C is given by Bolton as
where T is temperature in °C and vapor pressure is in kPa.
Iribarne, J. V., and W. L. Godson. 1981. Atmospheric Thermodynamics. D. Reidel, p. 65.
Bolton, D. 1980. The computation of equivalent potential temperature. Mon. Wea. Rev., 108. 1046–1053.
Ecuación de Clausius-Clapeyron[edit | edit source]
(También es conocida como ecuación de Clapeyron o ecuación de Clapeyron-Clausius). Es una ecuación diferencial que relaciona la presión de una sustancia con la temperatura en un sistema en el que dos fases de la sustancia están en equilibrio.
Dos expresiones generales son
donde p es la presión, T la temperatura, δs es la diferencia de entropía específica entre las fases, δv es la diferencia de volumen específico entre las dos fases y L es el calor latente del cambio de fase. La forma que se usa más en meteorología, que se relaciona con el cambio de fase entre vapor de agua y agua líquida, se obtiene después de algunas aproximaciones,
donde es es la presión de saturación del vapor del agua, Lv es el calor latente de evaporación y Rv es la constante de los gases para el vapor de agua. Se puede obtener una relación similar para la presión de saturación del vapor en contacto con una superficie de hielo al sustituir el calor latente de evaporación por el de sublimación. Se pueden integrar estas ecuaciones para obtener relaciones explícitas entre es y T, considerando valores conocidos en algún momento. Las relaciones empíricamente más precisas difieren ligeramente de los resultados obtenidos de esta manera. Bolton proporciona una expresión que se considera precisa al 0.3 % para -35 °C < T < 35 °C
donde T es la temperatura en °C y la presión de vapor se expresa en kPa.
Term edited 1 June 2022.