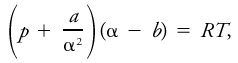Van der Waal's equation
From Glossary of Meteorology
The best known of the many laws that have been proposed to describe the thermodynamic behavior of real gases and their departures from the ideal gas laws.
It states
where a and b are constants dependent upon the gas, p the pressure of the gas, α its specific volume (measured in units of the specific volume of the gas at normal temperature and pressure), R the universal gas constant, and T the Kelvin temperature. Gas–liquid change characteristics and the properties of a substance in these two phases are predicted with some success by Van der Waal's equation, but not at all by the equation of state for perfect gases. When p is small and α is large, the correction terms are small and the two equations become identical.